

软质包装业务部
阿塔卡为客户提供订制的油墨、涂料和粘合剂解决方案,是软包装领域的领先者。
查看更多您已经订阅了我们的实时资讯。
act.page.footer.newsletter.message.success.unsubscribed
act.searchemptypage.button
Dear Customer,
TPE have a variety of properties or can be tailored and adapted to the requisite or desired characteristics depending on what end product is ultimately in focus. Accordingly, there is a range of solution options available for product design. Cooperation with ACTEGA and the PROVAMED® portfolio not only gives rise to very specific product benefits but also results in cross-product benefits.
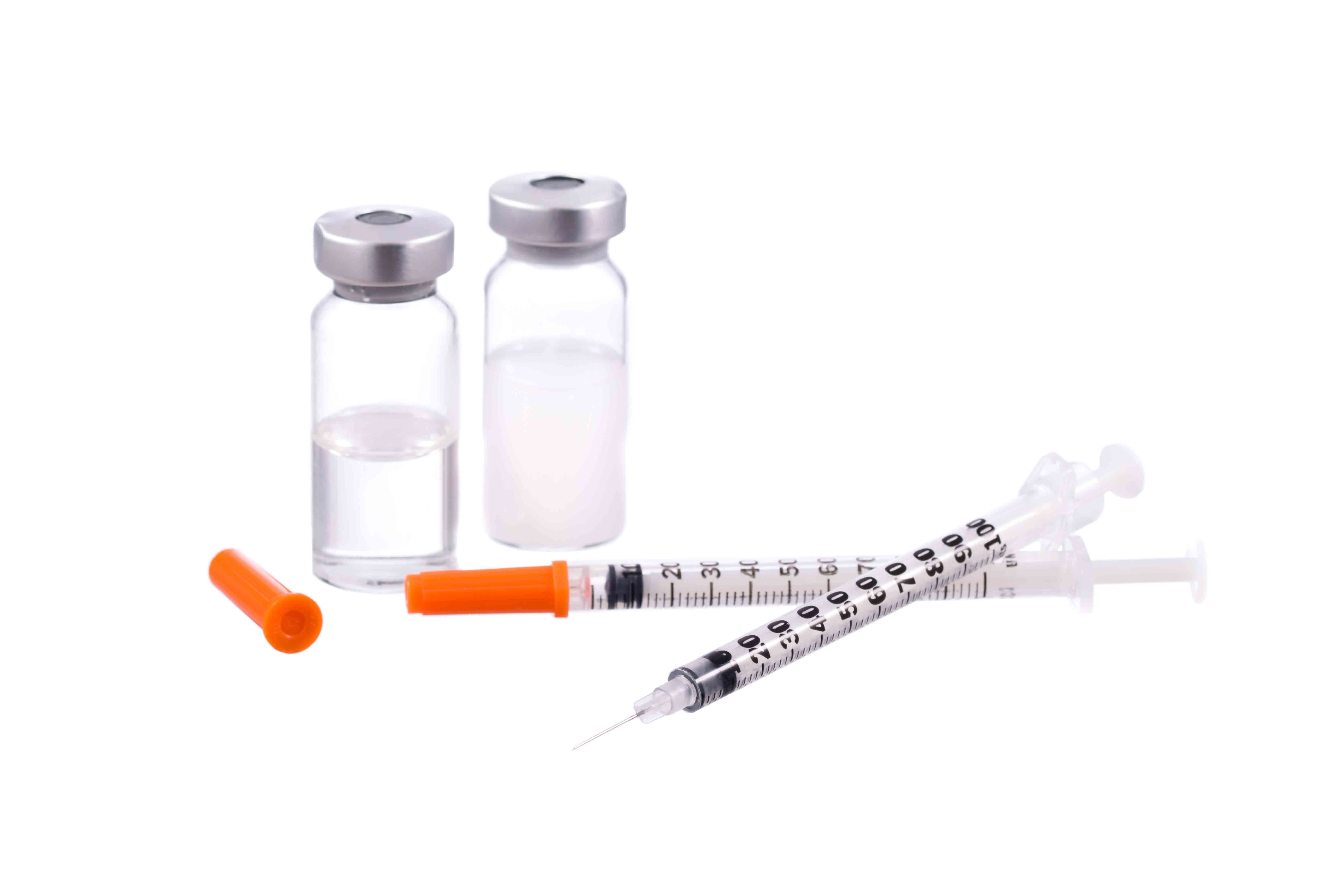
For example, in medicine and medical technology used for treatments where possibly “every second counts.” What is needed here is partners capable of delivering uninterrupted supplies of materials such as synthetic granulate for the production of medical products, thereby guaranteeing that no bottlenecks arise in the area of material supply. ACTEGA offers a remarkable range of services which guarantee the 100% deliverability of tailored PROVAMED®-TPE compounds for medical technology – at all times and to any destination. Enabling the manufacturers using these compounds to safeguard global healthcare with their products.
What’s more, manufacturers of medical technology face the difficult task of being obliged to subject their products to extensive regulatory qualifications and submit comprehensive documentation prior to launching them onto the market. This takes time and demands considerable know-how as well as a partner availing of exactly this expertise. At ACTEGA, therefore, there is a fixed contact person for each project who offers the corresponding service and specialist competence in the area of TPE materials and supports manufacturers and projects from development through to market launch.
Extensive and comprehensive documentation is available for the PROVAMED® TPE which guarantee compliance with the Medical Device Regulation as well as permit faster and better project success.
Another reason why comprehensive support is of importance is that not only the products themselves but often each individual component also needs to meet complex requirements with a current example being represented by injection and infusion systems.
According to USP Class VI, these also require biological testing and biological reactivity tests, among others. The biocompatibility of the compounds in accordance with ISO 10993 must be confirmed, whereby in vitro tests concern the cytotoxicity and viability of cell cultures. Biocompatibility means that the properties of the material used must be biologically compatible and should not display any undesirable interactions with other materials or living tissue. Apart from the general requirement concerning the use of source materials comprising non-toxic substances, this requirement is subject to additional specification for substances which are carcinogenic, toxic to reproduction, and affecting the endocrinal system.
Those manufacturers of medical technology relying on successfully tested, non-cytotoxic TPE synthetic granulates which can make an essential contribution toward successful qualification are significantly facilitated in terms of tasks relating to conformity. The biocompatible PROVAMED® TPE have already been tested to ISO 10993-5, with outstanding results. And they are free of PVC and plasticizers. The use of TPE materials also helps manufacturers of medical technology to counter the increasing criticism of PVC and plasticizers as well as improve the safety of patients and specialist personnel.
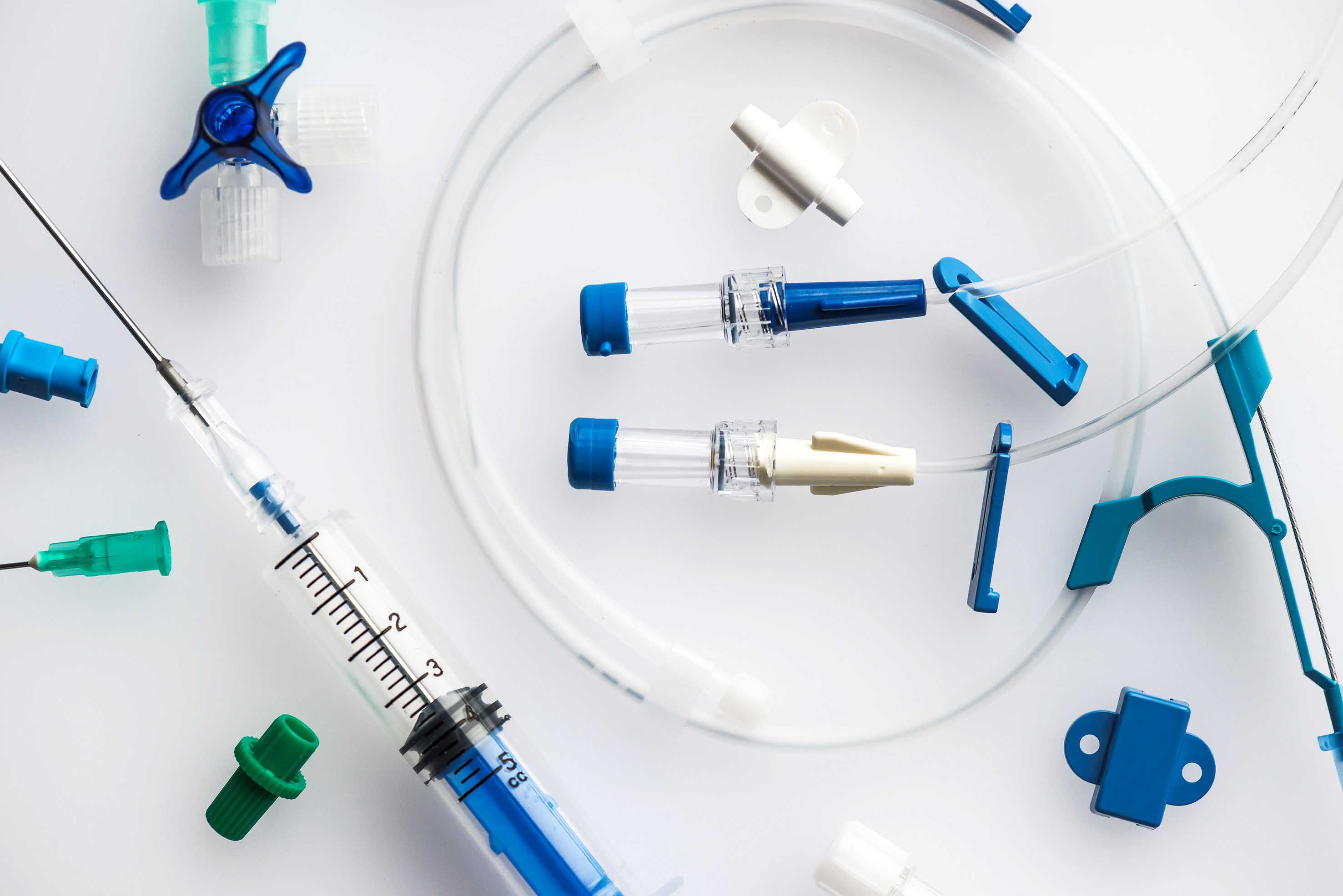
This safety aspect is also complied with by manufacturers of medical technology reducing the percentage of PVC in their products by combining components made of PVC with components made of TPE e.g. injection-molded soft PVC components with extruded TPE tubes, if we stay with the example of injection and infusion systems. This involves bonding the TPE to the PVC parts using conventional solvents, as is the case with our special PROVAMED® TPE variants. Unlike soft PVC, they do not contain any plasticizers with the result that they improve the safety aspect for hospital personnel, doctors, and patients. What's more, they also offer an extra degree of sustainability.
Those who wish to realize the advantages of the combination of two materials in a component in a single work step, thereby reducing the number of manufacturing steps, can avail of the economical two-component injection-molding process. This entails processing multi-component plastic items without any additional assembly in a single application which demands optimized adhesion on the part of the TPE. The adhesion-optimized TPE offered by ACTEGA permit shorter cycle times and extra wiggle room in price negotiations. As already outlined above, this is an aspect which cannot be ignored in our competition-driven environment.
Are you looking for your very own special customer benefits? Talk to us at any time!