

软质包装业务部
阿塔卡为客户提供订制的油墨、涂料和粘合剂解决方案,是软包装领域的领先者。
查看更多您已经订阅了我们的实时资讯。
act.page.footer.newsletter.message.success.unsubscribed
act.searchemptypage.button
Dear Customer,
The questions that are currently on everyone’s lips: When will there be a COVID-19 vaccine? Who will get it first? Will the vaccine be mandatory? If not, do I even want to be vaccinated?
According to the Robert Koch Institute, vaccines are among the most important and most effective preventative measures available in medicine. Accordingly, comprehensive vaccination programs since the middle of the 20th century have led to a huge reduction in various infectious diseases or even to regional or – as in the case of smallpox – global eradication thereof. The health authorities in the USA, the Center for Disease Control and Prevention (CDC), counts vaccination among the ten outstanding achievements by medicine and the public health sector. There is universal consensus among scientists, virologists, and the authorities that vaccination is the most important element of disposition prevention within general protection against infection.
By the time smallpox was eradicated in 1978 (also referred to as elimination of bacteria = full elimination of a pathogen), 375 million deaths had been recorded worldwide during the 20th century and in the USA alone, other infectious diseases which hardly ever appear nowadays were responsible for 39 million infections a year. According to the WHO and the Global Alliance for Vaccines and Immunization (GAVI), more than two million people still died of infectious diseases in 2002. Current estimates indicate that three children a minute (1.5 million p.a.) are still dying from infections which would be preventable by vaccination, whereby the group of anti-vaxxers is not exactly small.
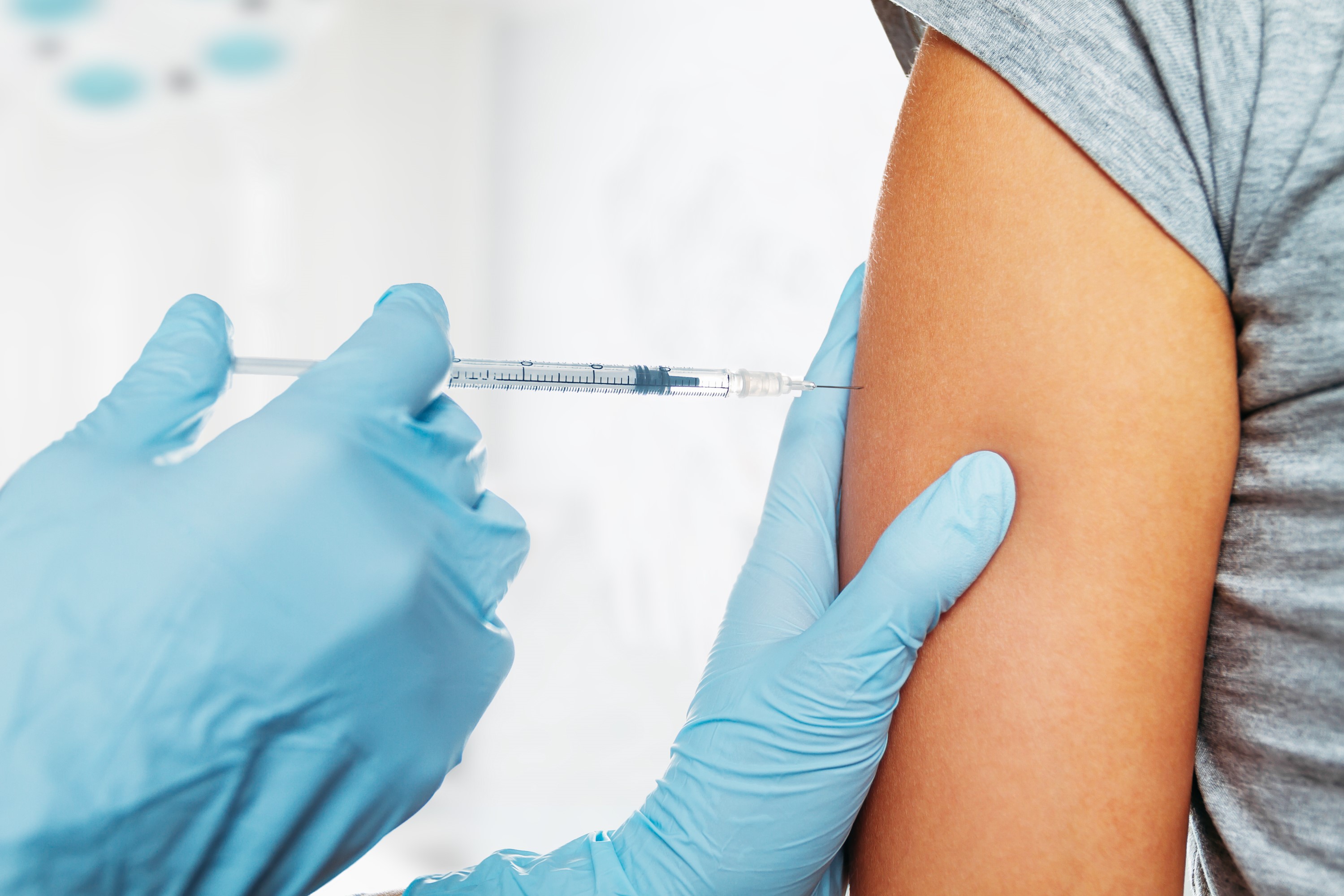
The main arguments against vaccination are often that the effectiveness of the vaccine cannot be proven, that vaccination does not provide long-term protection and therefore needs to be repeated constantly, that the vaccine makes recipients sick with the respective disease it is supposed to protect them from, that vaccines exacerbate allergies and that side effects and risks arise and/or cannot be assessed, that antibiotics can be relied on instead of vaccines but the pharmaceutical industry is only interested in lining its own pockets, that even doctors advise against vaccination and, finally, that vaccine production or administration could be susceptible to contamination which could lead to disease, and that diseases can be avoided and/or the risk of infection minimized with the right hygiene and nutrition. A long list of counter arguments is once again gaining in volume as the question looms as to whether it will come to mandatory vaccination if a vaccine becomes available against SARS-CoV-2 or the COVID-19 disease triggered by it. After not having mandatory vaccination in Germany for several decades, the gateway to further mandatory vaccinations was opened when mandatory vaccination against measles was agreed in November/December 2019 and will come into law as of July 31, 2021.
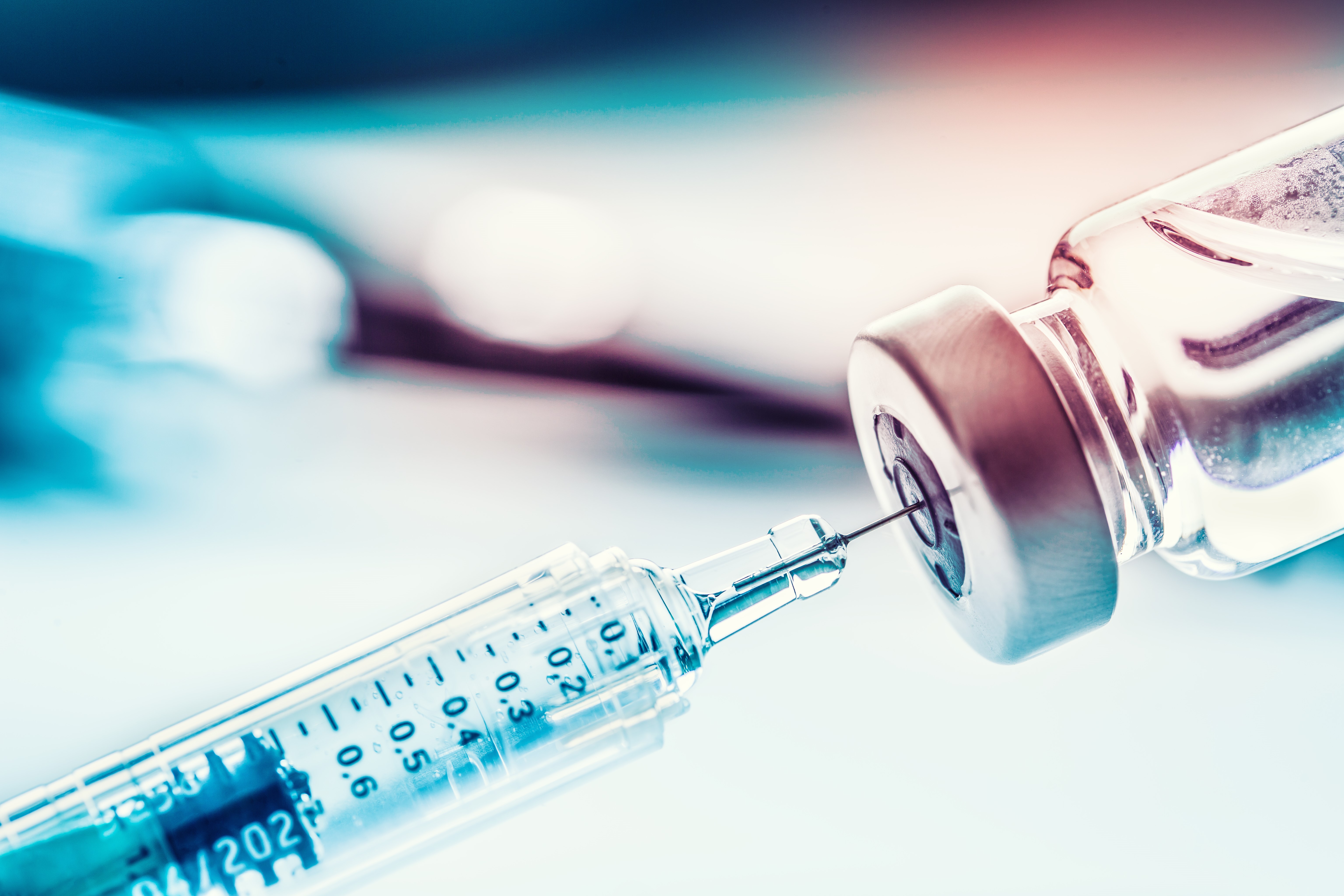
Whether on a voluntary or a mandatory basis, not only the right vaccine needs to be available but suitable tools also need to be in place and the corresponding material for manufacturing thereof. Now that oral vaccinations (e.g. against polio) entailing the administration of a live vaccine which, in turn, caused some infections, is no longer carried out, injections or cannulas are the remaining options for passive or active immunization which applies the vaccine intradermally (into the skin), subcutaneously (under the skin) or – as in most cases – intramuscularly (into the muscle).
With its PROVAMED® 6145 TL, 6245 NC and 6345 NC recipes, ACTEGA DS offers TPE specially for technical medical applications which demand sterilization of the end product. This has been proven by numerous tests before and after use of the various sterilization methods. The transparent and natural-colored variants of TPE compounds also comply with performance and conformity in accordance with USP 381. Furthermore, important factors such as easy processing, cost efficiency, low proportion of waste during production, and recyclability are also complied with.
PROVAMED® TPE can be used in a wide variety of customized requirement profiles. At ACTEGA DS materials are subject to further tests, e.g. on the topic of oxygen permeability, non-intentionally applied substances (NIAS) or allergens. The TPE are also free of PVC, phthalates, latex and ADC and do not require any cross-linkers such as rubber or vulcanized rubber. These can disintegrate which is why reaction accelerators (amines, sulfides, resin) are required to control such disintegration and can, in turn, lead to changes in the pH values of pharmaceuticals. In contrast, TPE require neither cross-linkers nor vulcanization which is intensive in terms of time and temperature.
If you would like to find out more about these or other TPE recipes, please do not hesitate to contact us.
As you know, the Fakuma has been canceled for this year. However, it takes place virtually and you can see the exhibitors' innovations in the online portal. Under this link you can see which highlights we are presenting:
https://www.fakuma-messe.de/nc/en/fakuma-virtual/showroom/exhibitor-virtual/48202/
We will also set up an electronic press box from the beginning of October. Various applications are described in detail therein. We will send you the link in time.