TPE for Reclosing Intravenous Bag Closure
They can be found in hospitals and doctors’ surgeries all over the world: intravenous bags. They have established themselves in the area of medical care and are very popular among doctors and nursing staff. And not without reason: they are easy to handle, intravenous bags deflate in full when empty, the drip chamber cannot run dry, and ventilation is not necessary. Consequently, pressure infusion is achieved swiftly.
Unlike once-off injections, infusion therapy refers to continuous, usually parenteral administration of medical fluids, primarily administered intravenously. Infusions are indicated to replace fluids or as volume replacement or substitution in volume and osmotherapy. Apart from fluid therapy, infusion solutions can also be found in the area of parenteral nutrition and as carrier solutions requiring a minimum duration of administration or when certain active agent combinations should not be exceeded. This is the case in electrolyte and chemotherapy, for example, or in the field of acid-base balance and the administration of antibiotics.
Do you have any questions? Contact us and we will be happy to advise you on your individual requirements.
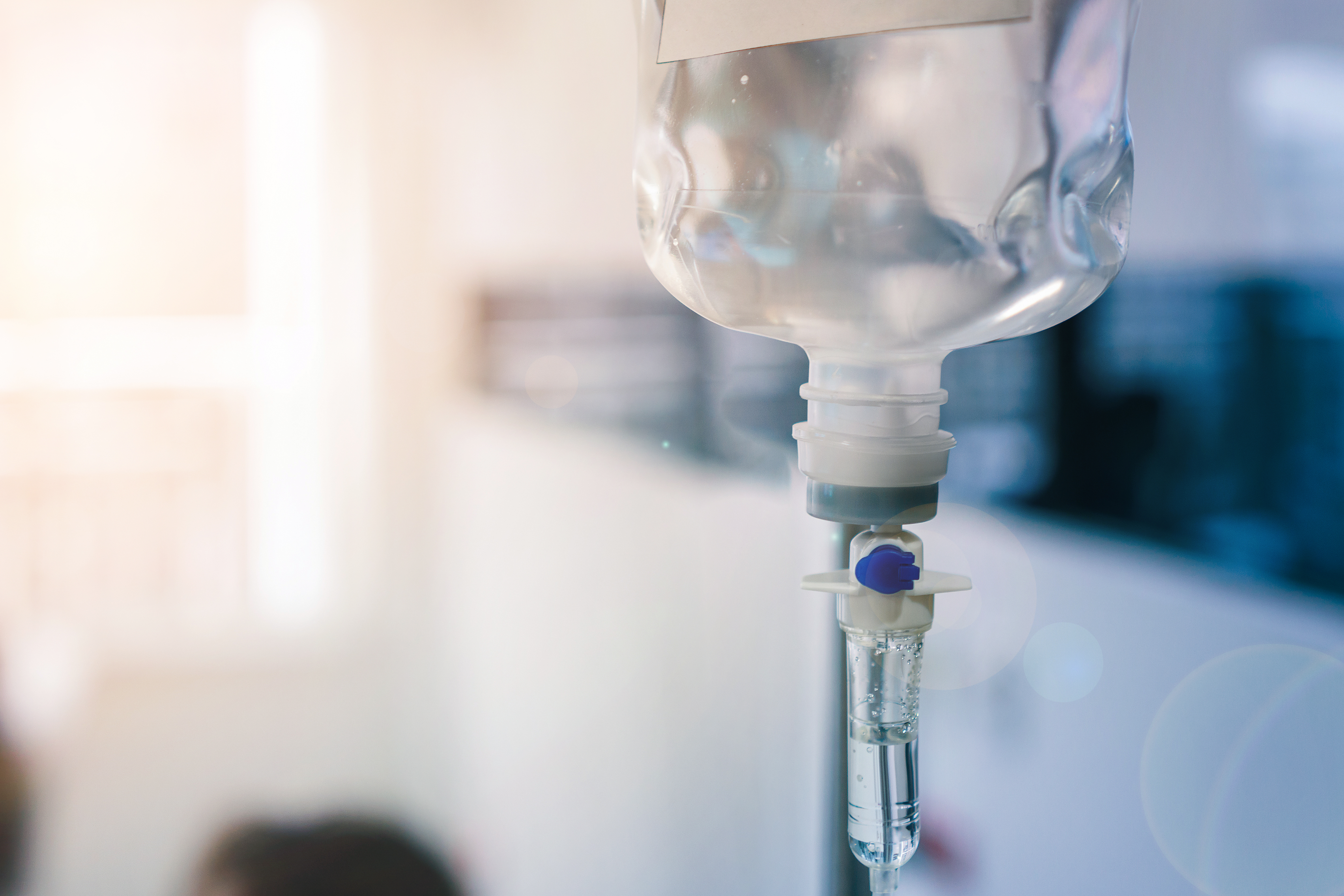
At ACTEGA, we exploit this variety displayed by thermoplastic elastomers for our PROVAMED® portfolio whose material properties can be flexibly classified within a wide framework. This means not only can it be tailored to your specific needs but also complies with the various requirements stipulated by stringent international rules governing technical medical products and therefore the actual raw materials themselves. Let’s take as an example material for packaging with direct contact to medical media – high-quality intravenous bag closures or so-called resealing membranes for intravenous bags or bottles. The septum used for connecting the tube to an intravenous bag is often made of rubber. But thermoplastic elastomers as an alternative have already yielded some convincing results. They are particularly efficient and hygienic in terms of processing. The material only comprises raw materials which meet the specifications of the medical market in order to eliminate the possibility of contamination.

Efficient and safe solution for medical use
These TPE can be sterilized and, unlike a rubber septum, offer the advantage of 2-component injection-molded processing. This dispenses with manual assembly of the rubber closures in the intravenous bag, which, in turn, saves time and reduces the risk of error. Furthermore, no costs arise for storage of the components and the possibility of contamination of the septum is also eliminated. Because TPE materials are so highly elastic, the septum reliably seals off the intravenous bag when the drip tube is removed. The actual production process is medically safe. As TPE materials are non-interlinking, the risk of chemical reactions and reactive residue by harmful chemicals in the rubber is effectively counteracted which, in turn, contributes to a maximum level of hygiene in this sensitive medical area.

Popular in medical care
Not only due to the rapid advancement of new highly-effective medication, particularly in the area of oncology, alternative materials free of plasticizers were sought for both intravenous bags and for their closures and filling and drip tubes. On the one hand, compatibility problems can arise with the material if it is manufactured from soft PVC. On the other hand, undesirable “side effects” can present if the infusion solution causes plasticizers and other additives to leach from the PVC material. This arises when the infusion solution contains fatty or lipid-like substances. Efficient solutions have been found here in the form of the TPE solution for intravenous bag closures or a multi-layer solution for filling tubes (e.g. EVA inner layer and TPE outer layer for two-layer tubes and soft PP/PP/TPE for three-layer tubes).
One step ahead
In a phase in which the demand for high quality is coupled with the necessity for drastic cost reductions, i.e. the phase in which the entire healthcare and medical sector currently finds itself, manufacturers of medical technology are relying heavily on answers from the supplier sector. There are hopes for solutions concerning existing and anticipated legal specifications in particular. Specializing in highly-sensitive areas of application and strongly-regulated markets, we offer a wide portfolio of efficient compounds for use in the medical environment. As a supplier of innovative, flexible plastics, we meet the requirements for ultra-durable, tailored materials while simultaneously adhering to regulatory stipulations. We make every effort to remain a step ahead of developments in this area. The PROVAMED® product range meets the highest requirements on purity, cleanliness and hygiene. The production of PROVAMED® granulate entails the use of raw materials which correspond to the requirements of the European Pharmacopeia or have been reviewed for bio-compatibility in line with generally accepted standards. The suitability of our plastics for sensitive applications has been confirmed by tests in accordance with ISO10993 and USPVI. Our TPE portfolio is free of latex, silicone and phthalates. These materials can also offer particularly good adhesive properties when bonded with thermoplastics such as PE, PP, PS, ABS, PC and PA, which are also maintained even in the case of continuous media contact and increased temperatures.
Our brands for medical TPE
PROVAMED®
Portfolio of standard products for medical technology and for customized TPEs for highly-sensitive areas of application.